Efficiency of detecting radioactivity
Efficiency is the fraction of the radioactive disintegration that are detected by the counter. Determine efficiency by counting a standard sample under conditions identical to those used in your experiment.
With 125I, efficiencies are very high, over 90%. It depends on the geometry of the counter. The detector doesn't entirely surround the tube, so a few gamma rays (photons) miss the detector.
With 3H, the efficiency of counting is much lower, often about 40%. The low efficiency is mostly a consequence of the physics of decay, and can not be improved by better instrumentation or better scintillation fluid. When a tritium atom decays, a neutron converts to a proton and the reaction shoots off an electron and neutrino. The energy released is always the name, but it is randomly partitioned between the neutrino (not detected) and an electron (that we try to detect). When the electron has sufficient energy, it will travel far enough to encounter a fluor molecule in the scintillation fluid. This fluid amplifies the signal and gives of a flash of light detected by the scintillation counter. The intensity of the flash (number of photons) is proportional to the energy of the electron. If the electron has insufficient energy, it is not captured by the fluor and is not detected. If it has low energy, it is captured but the light flash has few photons and is not detected by the instrument. Since the decay of many tritium atoms does not lead to a detectable number of photons, the efficiency of counting is much less than 100%.
Efficiency of counting 3H is reduced by the presence of any color in the counting tubes, if the mixture of water and scintillation fluid is not homogeneous, or if the radioactivity is trapped in tissue chunks (so emitted electrons don't travel into the scintillation fluid).

Specific radioactivity
When you buy radioligands, the packaging usually states the specific radioactivity as Curies per millimole (Ci/mmole). Since you measure counts per minute (cpm), the specific radioactivity is more useful when stated in terms of cpm. Often the specific radioactivity is expressed as cpm/fmol (1 fmol = 10-15 mole).
To convert from Ci/mmol to cpm/fmol, you need to know that 1 Ci equals 2.22 x 1012 disintegrations per minute. Use this equation to convert Z Ci/mmole to Y cpm/fmol when the counter has an efficiency (expressed as a fraction) equal to E.
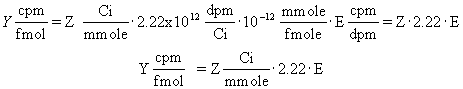
Note: You may also encounter the unit Becquerel, which equals one radioactive disintegration per second.

Calculating the concentration of the radioligand
Rather than trust your dilutions, you can accurately calculate the concentration of radioligand in a stock solution. Measure the number of counts per minute in a small volume of solution and use this equation. C is cpm counted, V is volume of the solution you counted in ml, and Y is the specific activity of the radioligand in cpm/fmol (calculated in the previous section).
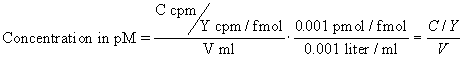

Radioactive decay
Radioactive decay is entirely random. A particular atom has no idea how old it is, and can decay at any time. The probability of decay at any particular interval is the same as the probability of decay during any other interval. If you start with N0 radioactive atoms, the number remaining at time t is:
Kdecay is the rate constant of decay expressed in units of inverse time. Each radioactive isotope has a different value of Kdecay.
The half-life (t½) is the time it takes for half the isotope to decay. Half-life and decay rate constant are related by this equation:
This table shows the half-lives and rate constants for commonly used radioisotopes. The table also shows the specific activity assuming that each molecule is labeled with one isotope. (This is often the case with 125I and 32P. Tritiated molecules often incorporate two or three tritium atoms, which increases the specific radioactivity.)